PMTA simply stands for Pre-Market Tobacco Product Application, is an application required by FDA to all manufacturers of tobacco and new tobacco products to file for an approval in order to legally continue marketing and selling their products in the US markets.
A new tobacco product is either a product commercially marketed in the United States after February 15, 2007, or any modification to a tobacco product commercially marketed after February 15, 2007.
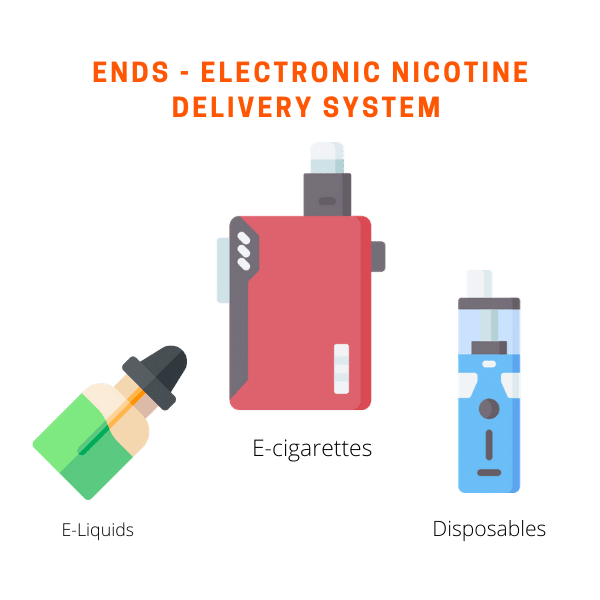
ENDS, short for Electronic Nicotine Delivery System, falls under the definition of new tobacco products therefore all ENDS manufacturers must file for market approval to continue to stay in business. FDA categorizes ENDS to include E-Liquids, E-Cigarettes, and ENDS products that package e-liquids and e-cigarettes together.
PMTA is not only to seek marketing authorization, but the objective of PMTA for ENDS lies in the interests of public health. FDA wants all tobacco and new tobacco products sold in the US market to be regulated to ensure safety and security for both manufacturers and consumers of these products, as well as the protection of the public health as a whole.
In order to do that FDA needs to assess the risks resulting from its usage or effects of its usage to both users and non-users of the products, its marketing and promotional activities, as well as the manufacturing processes and quality standards of the products sold.
Due to little information and scientific research about the ENDS industry, it is difficult for regulatory bodies like FDA, to provide substantive review for these products and devise ways to formally regulate its usage and marketing activities in the long term.
Therefore, manufacturers filing these data to FDA will be crucial in ensuring continuity of this industry as a properly regulated industry that ensures not only protection of the public health, but also quality products that conforms to global standards and consumers safety usage are marketed in the US.

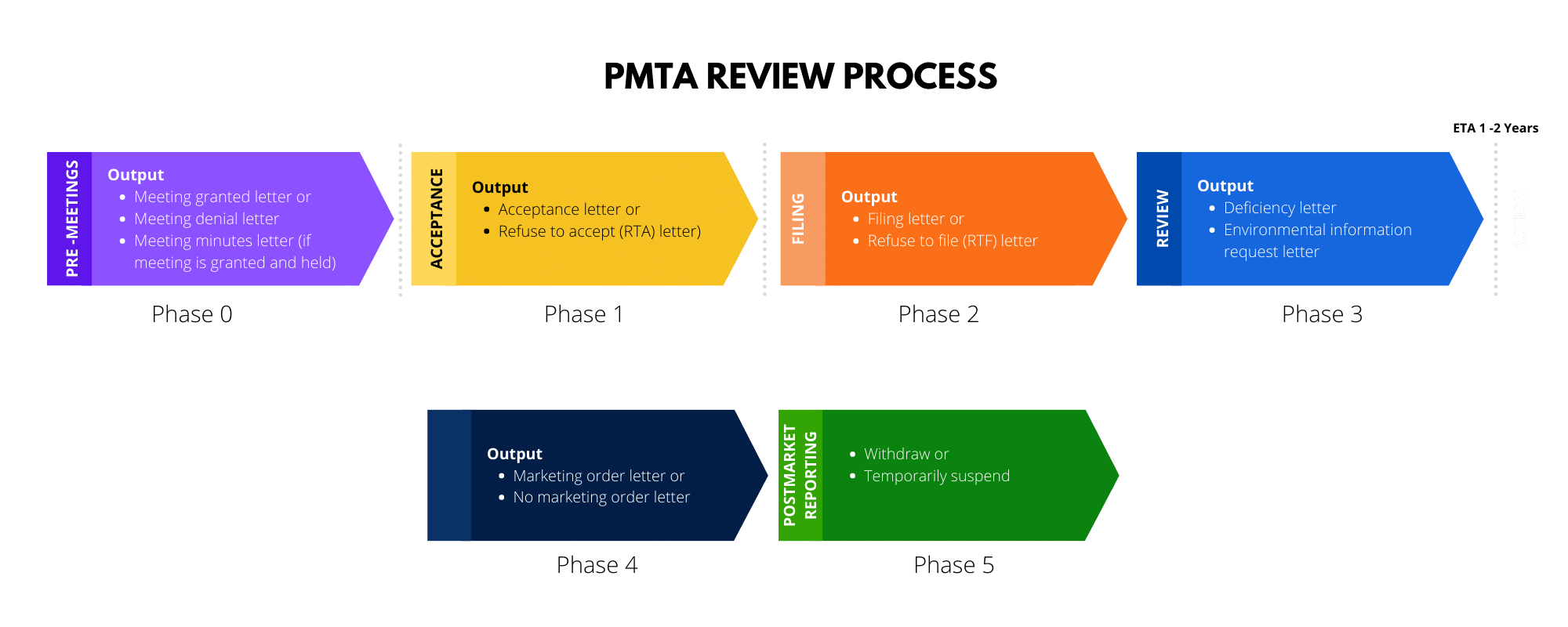
To obtain this marketing authorization, FDA has devised steps and required processes to ensure applications submitted and it’s required information are eligible for FDA to conduct substantial review.
The process is extensive but can be broken down as follows:
The required information needed for PMTA application in order to be accepted for PMTA review can be generalized into:
Product Information and Design
Manufacturing Practices and Quality
Clinical Studies and tests
Non-Clinical Studies
FDA estimates that the approval and the process needed to conduct the PMTA substantive review will take approximately 1-2 years.
Aspire understands the importance of PMTA for the vaping industry and the public health, and is highly committed to comply with PMTA. We have shown compliance with TPD for all of our products and will provide the same commitment for PMTA. We have submitted our first application to acquire marketing authorization for our popular products and will continue to do so for all future products.
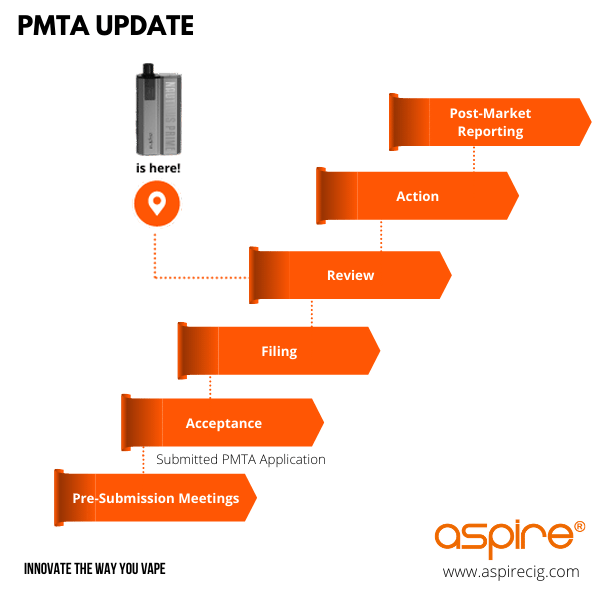





Aspire understands the importance of PMTA for the vaping industry and the public health, and is highly committed to comply with PMTA. We have shown compliance with TPD for all of our products and will provide the same commitment for PMTA. We have submitted our first application to acquire marketing authorization for our popular products and will continue to do so for all future products.

March 18, 2021 Aspire is proud to announce we have received PMTA Acceptance letter from the FDA on March 18, 2021. We have reached a step closer to meeting FDA criteria for a full scientific review before moving onto the next step of the PMTA process.
September 9, 2020 Aspire successfully submitted premarket tobacco application(PMTA) in Time.The documentation includes a broad and exhaustive investigation with scientific evidence of the quality and safety of our electronic nicotine-delivery systems (ENDS).
Aspire believes in normal and logical regulations to protect the people against harmful tobacco products. We also believe that the ENDS industry should offer high-quality products that comply with health and marketing standards.
Sign up for special offers and updatesfrom aspire now!
UNLOCK OFFERBy signing up, you agree to receive email marketing. Offer is only available for first-time subscribers who have not placed an order with us before.